Menu
Home / پزشکی و بهداشتی / ضدعفونی کننده های پوست و جراحی / Septiprep plus
the amount of the compound in 100 grams of the product:
Spray an adequate amount of the solution onto the injection site and allow it to dry after spraying. If there is any visible contamination, clean the injection site from the center outward.
* This product is suitable for disinfecting the skin for various types of injections, including intravenous (IV), intramuscular (IM), and subcutaneous (SC) injections.
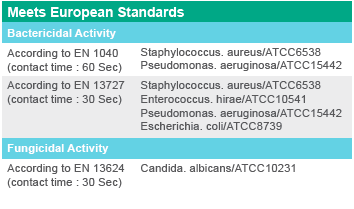
* The spectrum of antimicrobial effect is regularly updated by the scientific department of the company. Therefore, to learn about the latest approved product standards, please contact the company’s authorized representatives.

Behban Shimi founded one of the most reliable and largest factories for the production of disinfectants, antiseptics and exclusive cleaners in the Middle East region in 2001. This company currently produces and supplies a wide range of products related to increasing safety and health to regional markets.
Contact us
All rights of this website belong to Behban Farmed Lotus Company. 1377-1402