Menu
Home / Medical & public / Hemodialysis disinfectants / Percidine 3%
After placing the requested container in the special place of the hemodialysis machine for the disinfectant solution, connect the relevant connections and pipes to it according to the instructions of the machine user (in different brands, the connection method may be different). Machine settings for cold disinfection with Prostatic Choose the acid. Typically in different brands of the machine with a concentration of 3-5% for a cycle of 30-50 degrees Celsius. Possible residual disinfectant solution in the wash water with the help of potassium iodide and starch glue (iodometry method) can be detected.
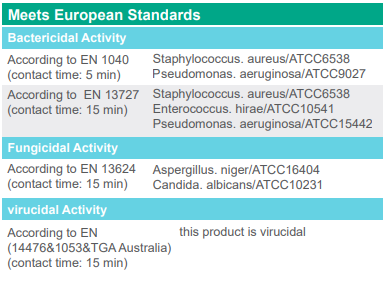
* The range of antimicrobial effects is regularly monitored and updated by the company’s scientific department, so please contact the company’s authorized representatives to learn about the latest approved standards of the product.

Behban Shimi founded one of the most reliable and largest factories for the production of disinfectants, antiseptics and exclusive cleaners in the Middle East region in 2001. This company currently produces and supplies a wide range of products related to increasing safety and health to regional markets.
Contact us
All rights of this website belong to Behban Farmed Lotus Company. 1377-1402